Choroideremia (also spelt choroideraemia) is an X-linked inherited retinal disease (IRD). This means that males are most affected (because females have two copies of an X-chromosome – see inheritance patterns for more details). Although sometimes, female carriers (who only have one copy of the mutated gene) can also experience symptoms of varying degrees.
Like retinitis pigmenosa (RP), the vision loss in choroideremia is due to damage to the light-sensitive photoreceptor cells in the retina. However, in choroideremia this happens following damage to the choroid, a network of tiny blood vessels behind the retina. The vision loss from tends to progress over time, but the rate change can vary between two individuals.
How is choroideremia identified?
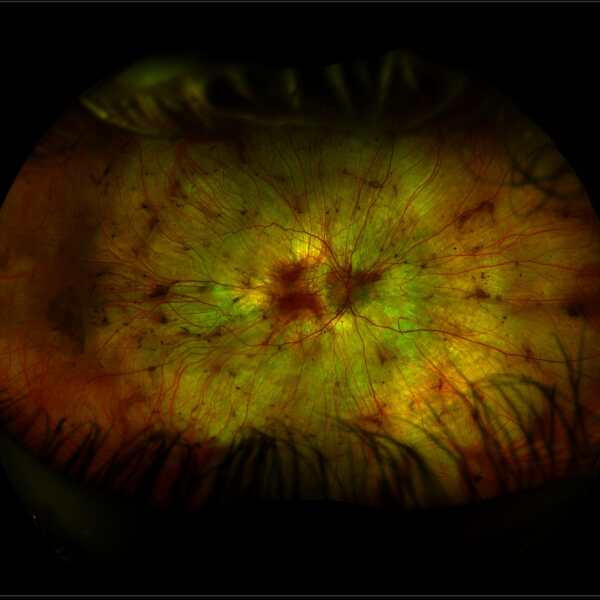
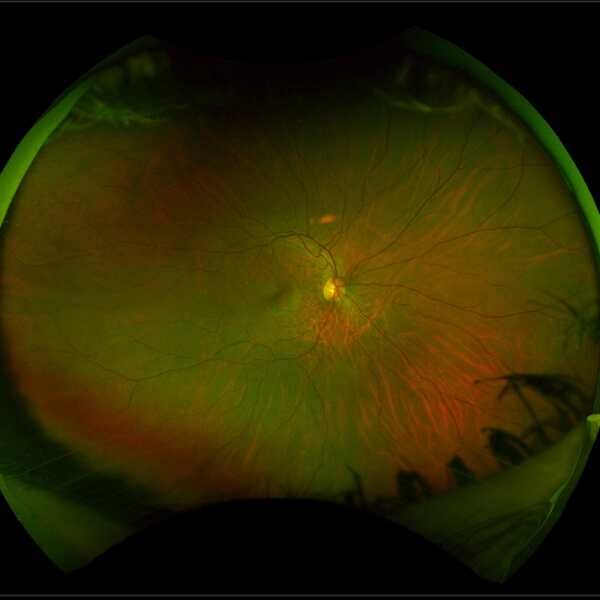
Choroideremia has a distinctive appearance at the back of the eye, due to the thinning of the choroidal layer. The appearance of a retina with choroideremia (image below left) shows a white patchy appearance, with small regions of healthier tissue remaining as pink areas. This compares to a healthy retina (image below right).
A family history suggesting an X-linked inheritance pattern will also help an ophthalmologist to make the diagnosis. This diagnosis can then be confirmed with genetic testing – the causative gene is called CHM.
Source: Images taken on a wide-view fundus camera (Optos) and are courtesy of the VENTURE study.
What are the symptoms of choroideremia?
The early symptoms of choroideremia are similar to RP, with poor night vision and “clumsiness” often being reported first. These symptoms occur because the rod photoreceptors are damaged before the cone photoreceptors in this disease.
In later stages of the disease, vision loss will move towards the centre, causing tunnel vision. Generally, central vision is maintained until late in life.
What is the cause of choroideremia and how is it inherited?
Unlike some other retinal degenerations, such as RP, cases of choroideremia are due to mutations in just one gene, known as CHM.
It is genetically passed through families by X-linked inheritance. Therefore, females are carriers of the condition, but do not generally display the severe symptoms of the disease. Males only have one X chromosome and will become affected by the condition if he receives a faulty copy of the gene. Affected males cannot pass on the disease to their sons, because they pass on their Y chromosome. Men with choroideremia must pass on the disease gene to all of their daughters, who then become carriers of the gene.
If a family member is diagnosed with choroideremia, it is strongly advised that other members of the family also have an eye exam by an eye doctor (ophthalmologist) who is specially trained to detect retinal diseases.
What treatments are available
There are currently no treatments available but a number of technologies are in research and development stages – see emerging treatments.
