One approved gene therapy
There is only one inherited retinal disease (IRD) gene therapy available commercially – voretigene neparvovec-rzyl (Luxturna®). This gene therapy targets mutations in the RPE65 gene which may be associated with retinitis pigmentosa or Leber congenital amaurosis (LCA). As of August 2025, only 20 people in Australia have received the treatment. However, the success of this first IRD gene therapy has opened the door for others to come, with several trials underway at present.
If you would like to know if gene therapy is an option for you, or your family member, please speak to your ophthalmologist.
What are genes?
The “recipe” for the proteins that make up the human body is coded in molecules called DNA, which form long strands called chromosomes.
Within the chromosomes, small sections of these DNA molecules group together to form a gene, which is responsible for one specific protein.
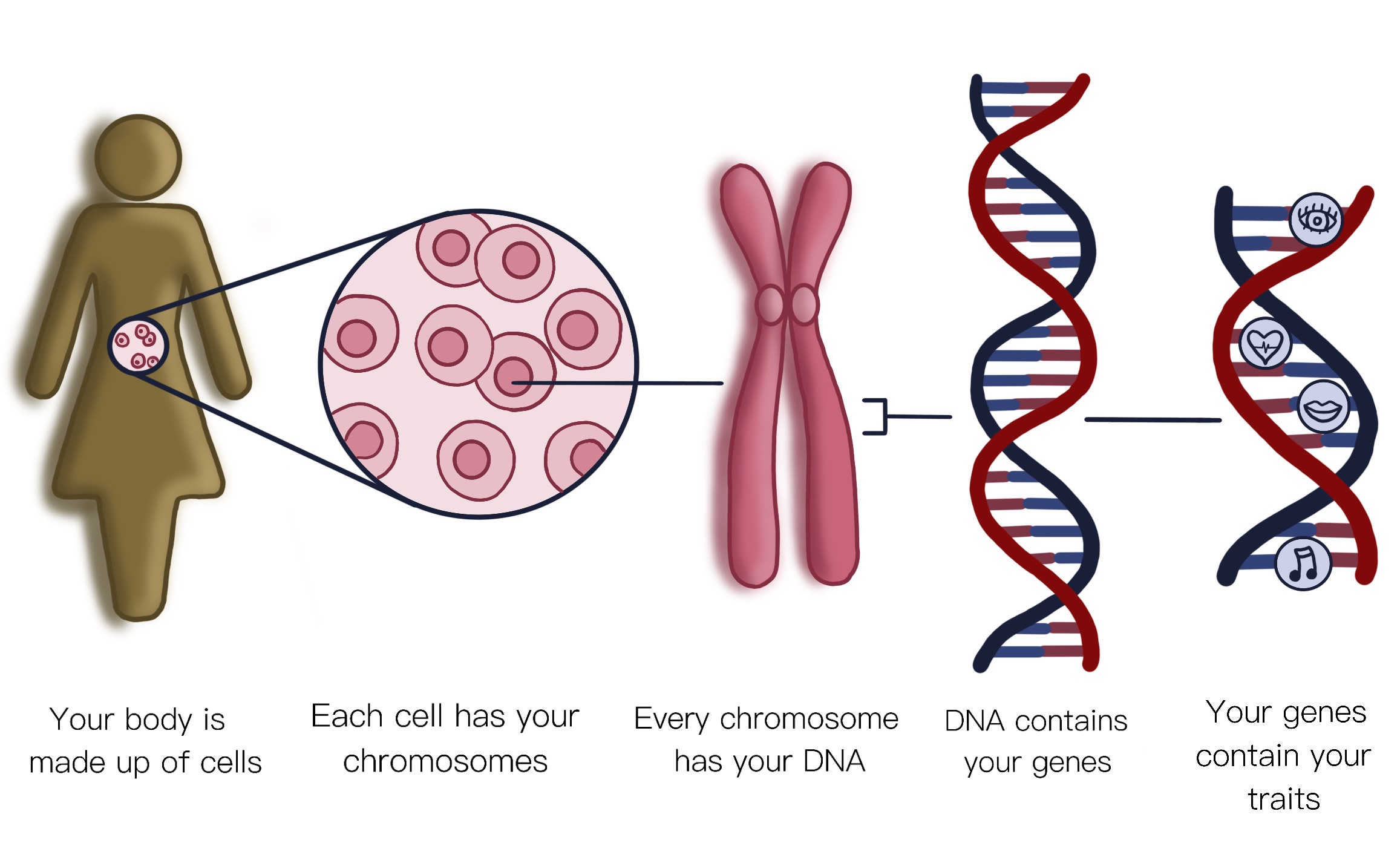
- The proteins coded by genes build, regulate and maintain your body. For instance, they build bones, they enable muscles to move, they control digestion, and they keep your heart beating. It is thought that we have about 20,000 genes in our cells. They make us what we are.
- Genes are inherited from our parents. We inherit a set of chromosomes from each parent – 23 from our mother, and 23 from our father. Two of the chromosomes (the X and the Y chromosome) determine your sex as male or female when you are born.
- Unfortunately, DNA can be damaged, leading to “gene mutations”. These mutations can cause disease which, depending on the type of gene affected, can then be passed on to future generations within a family.
IRDs are usually monogenic conditions – this simply means that a mutation in one gene is enough to cause the condition. This makes them an excellent candidate for gene therapies, which aim to correct these genetic errors.
What is gene therapy?
Gene therapies may treat inherited retinal disease by repairing or replacing abnormal genes.
A gene mutation can cause different problems in the eye. Sometimes, the mutation stops a protein from being made, which is called a “loss of function” error. In these cases, gene therapy can allow a healthy copy of the gene to be inserted into the eye, to compensate for this mutated copy.
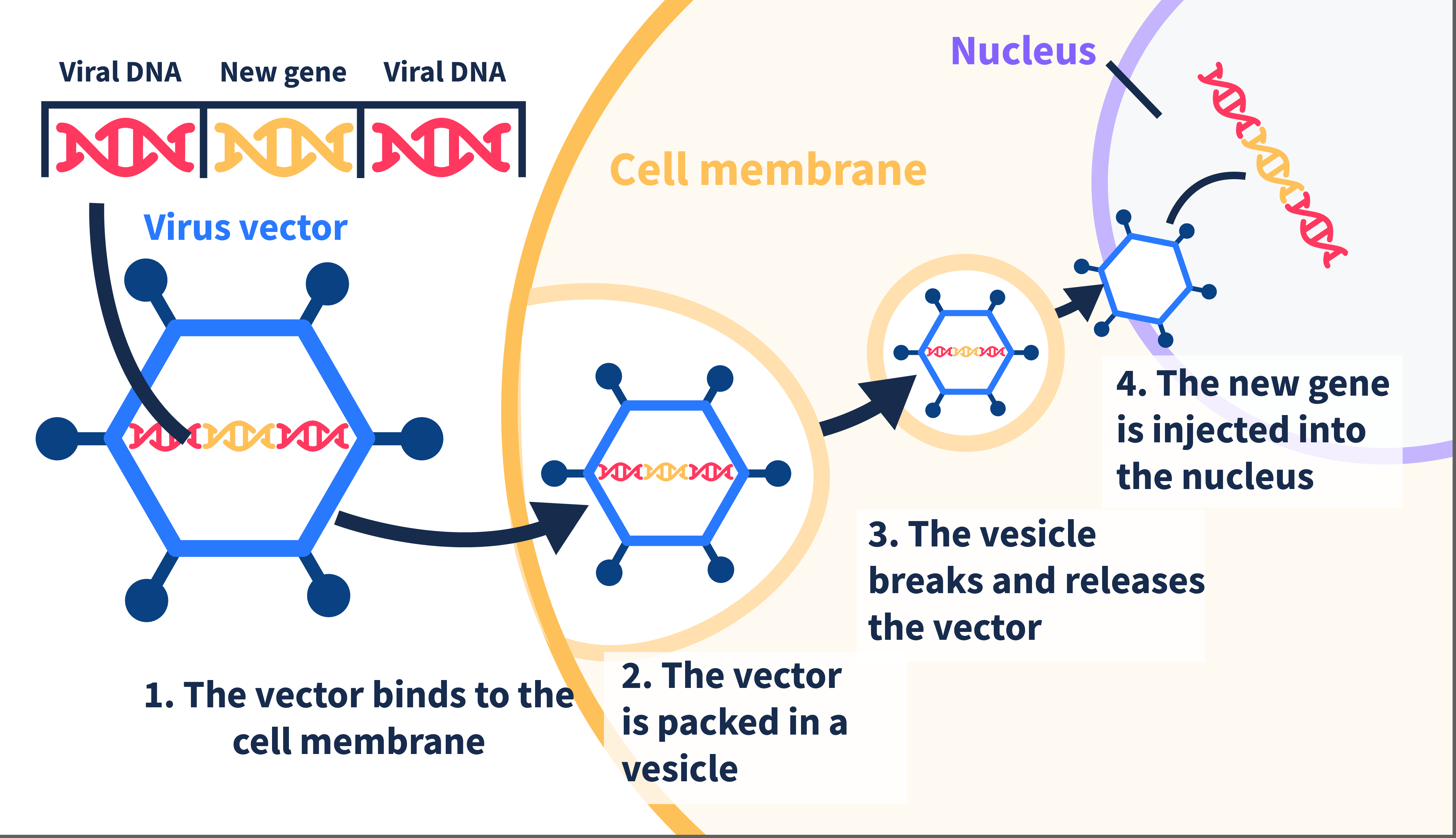
To do this, most gene therapies use a virus to carry the gene into the eye.
- This virus is very similar to the common cold, but it is altered so that it cannot cause disease. It simply acts as a transport vehicle for the gene.
- The correct version of the gene is placed inside the virus, which is then injected into the eye.
- Once in the eye, the virus spreads and transfers the healthy gene into retinal cells, hence helping to restore function to those cells.
- Current research is underway to improve the efficiency of this process, looking at different types of viruses, or even other ways of transporting the genes (including fat molecules, or incredibly small particles of metals like gold).
In other cases, the gene mutation causes an “up-regulation” of the protein being made, so the gene therapy needs to stop that gene from working so hard. In this case, therapies like gene editing can work, where the mutated gene is removed, and replaced with a healthy version.
There are various forms of gene therapy being developed. Some are developed specifically for one gene mutation; for example, the only commercially available IRD gene therapy, Luxturna™, is for mutations in the RPE65 gene. Other gene therapies are designed to work for a range of different IRDs and are not specific to the gene affected.
What are the main types of gene therapy?
There are many different types of gene therapy being developed for IRDs, with different advantages and disadvantages to each. In simplistic terms, these can be considered in three main categories:
- Gene replacement therapy
- In the simplest form of gene therapy, an IRD is caused by a mutation in a single gene, which leads to insufficient function in the retinal cells.
- To compensate for this, a healthy version of the gene can be injected into the eye (normally in a viral vector), to replace the faulty version.
- This healthy gene will then cause production of the missing proteins, restoring function to the retina.
- This technique is also known as gene augmentation therapy.
2. Gene editing therapy
- In some IRDs, the mutated gene is too large to fit into a viral vector, or the mutations may not be amenable to simple gene replacement therapy.
- In these, one option is editing the gene in the cell.
- This is achieved using a technique called CRISPR, which allows identification of the problematic region of DNA, and then altering that code.
- CRISPR can be used to turn on or off genes or change other genes to compensate for the fault.
3. Gene-specific targeted therapy
- In this category, treatments may include a combination of genes – some to replace non-working genes, and others to silence overactive genes.
- These targeted therapies can be designed for the DNA genetic code, or the RNA transcripts which are produced during cell function.
- To understand the difference between DNA and RNA, we can think of DNA as the master blueprint of a building, storing all the information needed for construction. RNA is like a copy of specific sections of that blueprint. It’s made when the cell needs those instructions for doing something, like building a protein.
- RNA is essential because it helps translate the information in our genes into actions or products that our cells need to function properly. It’s like a mediator, ensuring that the information coded in our DNA is correctly used to build and maintain our bodies.
Optogenetics
Optogenetics uses gene therapy and/or drugs to change the function of remaining cells in the retina, making them become light sensitive hence improving vision. These treatments are currently aimed at people with very poor residual vision.
What IRD gene therapies are currently available?
Only one gene therapy available
As of October 2024, there is only one IRD gene therapy available commercially – voretigene neparvovec-rzyl (Luxturna®). This drug targets mutations in the RPE65 gene, which affects around 2% of people with retinitis pigmentosa. Candidates for this treatment normally have a subtype of RP called Leber congenital amaurosis, which causes severe vision loss in early childhood. It is thought that this therapy will be suitable for less than 100 people in Australia.
Luxturna™ was approved by the USA Food and Drug Administration in 2017, and in Australia by the Therapeutic Goods Administration in 2020. There are currently two treatment sites in Australia – the Sydney Eye Hospital (team led by Professor John Grigg & Dr Matthew Simunovic) and the Royal Victorian Eye and Ear Hospital (team led by Dr Thomas Edwards and Associate Professor Fred Chen). As of October 2024, 14 patients in Australia have been treated with this gene therapy.
What does a gene therapy surgery involve?
The currently available Luxturna® gene therapy is delivered to the eye in a subretinal injection. The procedure is done in an operating theatre, where a highly skilled surgeon will inject the drug underneath the retina. The treatment is one-off, with the benefits hypothetically lasting for life (noting that these trials only started 15 years ago, so we don’t have long term data at this time).
The aim of a gene therapy treatment is to STOP progression of the disease. In some cases, the treatment can lead to improvements in vision, but this is not the primary aim. As such, it is thought that treating people at earlier stages of disease will have the best outcomes for gene therapy. Overall, outcomes for people receiving Luxturna® have been impressive, with some people regaining significant vision. Research is continuing to follow people treated with Luxturna, to see what the long term effects of treatment are.
What other gene therapies are in development?
A recent systematic review 1 showed that the highest number of scientific articles on gene therapy have been published for (in order):
- RPE65 associated IRD (retinitis pigmentosa and Leber congenital amaurosis) – this is the gene for which Luxturna™ has been approved.
- Leber hereditary optic neuropathy (LHON) – due to mutations in the ND4 gene
- Choroideremia – due to mutations in the CHM gene
- X-linked retinitis pigmentosa – due to mutations in the RPGR gene
- Stargardt disease – due to mutations in the ABCA4 gene
There are clinical trials underway for these and other IRDs. To find out more about current and emerging clinical trials, please see our “Clinical Trials” section.
To investigate the perspectives of people with IRDs on these emerging gene therapies, including identifying any barriers for uptake, Retina Australia funded a large national survey in 2021, of almost 700 Australians with IRDs (or carers). A short report is here, showing that 92% of people with an IRD would be interested in accessing gene therapy, should it be available for them.
What gene therapy research has Retina Australia funded?
- 2023: Establishing novel AAV gene editing for Usher Syndrome
- 2023: Using RNA-silencing to tackle neuroinflammation in retinal degeneration
- 2022: RNA base editing strategies as potential therapeutic of inherited retinal dystrophies
- 2021: Strong, fast, then none: development of novel promoters for gene-editing therapies
- 2021: Potential Participant Perspectives in Ocular Gene Therapy in Australia
- 2019: Dual AAV retinal gene therapy approach for Usher 1F treatment
- 2019: Define the mechanism by which C1 rescues some functions in Best Disease RPE
- 2015: Correcting inherited retinal disease through gene editing
