21 April, 2024
Thanks to decades of eye research, we are making significant progress in our quest to find new treatments for inherited retinal diseases (IRDs).
Even though IRDs impact a relatively small segment of the population, they come in different forms, making it challenging to understand how treatments will work and what we should measure to see if they are effective in clinical trials.
Unfortunately, the lack of sensitive outcome measures has meant that some clinical trials of IRD treatments have not met their endpoints, and hence the treatment has been discarded.

Many researchers and clinicians in the field believe that standard visual tests, such as visual acuity, may not be sensitive enough to measure changes in IRD, and hence new endpoints are required in these instances.1
This article explores new imaging, vision testing and quality of life assessment methods that are currently being developed for IRD clinical trials. Exploring the intricacies of these tools, this article aims to shed light on what is being examined, measured, and the reasons behind these evaluations. For those who have undergone one of these tests, this information may offer a clearer understanding of the purpose and significance of the examination.
What are novel endpoints and why are they important for clinical trials?
The selection of appropriate criteria, referred to as endpoints or outcome measures in clinical trials, is crucial for evaluating how well a treatment achieves the desired therapeutic effects for a medical condition. Ideally, the endpoint used in a clinical trial should be easy to attain, consistent and reproducible in its results, and directly related to the patient’s well-being. In the context of IRDs, regulatory authorities worldwide, such as the USA Food and Drug Administration (FDA), currently have designated four key clinically relevant endpoints to be used as primary outcome measures in evaluating the efficacy of an intervention within a clinical trial. 2 3
The four clinically relevant endpoints focus on:
- Central vision (best-corrected distance visual acuity)
- The sensitivity of specific parts of the retina (visual field sensitivity)
- The response of the entire retina in detecting minimum light levels (full-field light sensitivity threshold testing), and
- The confidence, speed and safety with which participants can move around (mobility testing)
The challenge is that IRDs are generally slowly progressing, and so it is often difficult to see a difference in a measure during a clinical trial period (typically under two years). In addition, many IRD treatments being developed (such as gene therapy) have a primary goal to slow progression, not to improve vision. This means that the outcome measures used need to be able to differentiate between a slow deterioration and stable retinal function. This emphasises the importance of exploring novel, or new, advanced but non-invasive and precise ways to measure how well a treatment is working.4
What are some of the different technologies and novel endpoints that are accelerating IRD research?
1. New imaging techniques
Ophthalmology clinical trials have a particular advantage over many other drug trials – it is possible for us to directly look at the tissue being treated (i.e. the retina). Photographs and scans of the retina are now widely available both in clinical practice and in research settings. Some of the newer modalities are described below.
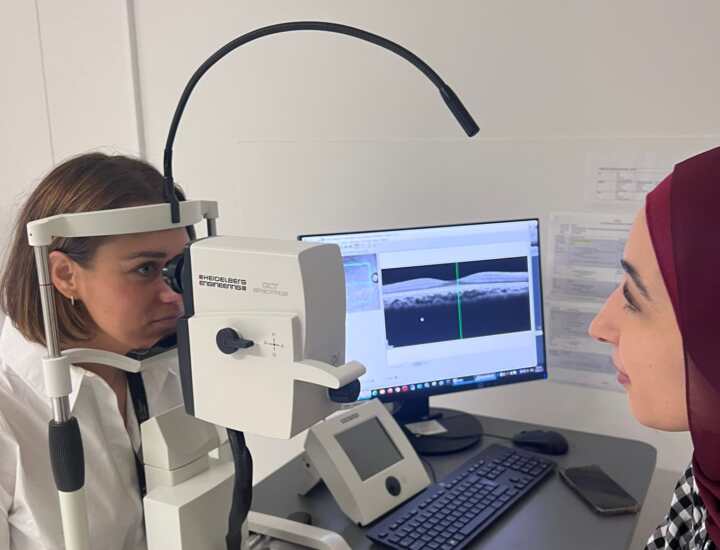
- Optical Coherence Tomography (OCT)
OCT (as pictured on the right) is a non-invasive imaging technology that utilises light waves to generate detailed, cross-sectional scans of the retina.
This method helps us to see the layers of the retina and take very exact measurements of the thickness of different layers. 5
Changes in those thicknesses and in structures identified in OCT scans can serve as endpoints, providing an objective reflection of structural changes and responses to treatments in IRDs.
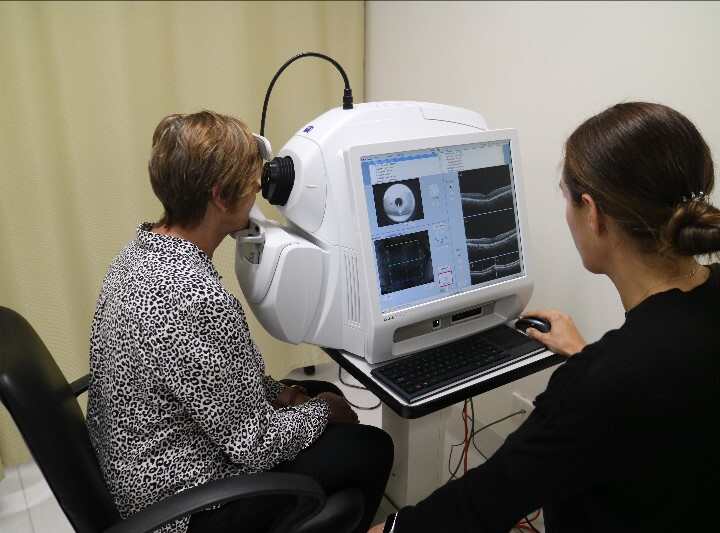
- Optical Coherence Tomography Angiography (OCT-A)
OCT-A (as picture on the right) is also a non-invasive and advanced imaging technology used to image blood vessels within the eye.
Previously, imaging the blood vessels required dye injections 6, but the OCT-A process is quick, accurate, and efficient, capturing high-resolution images of even the smallest blood vessels in the eye without the need for those dyes.
Changes in the blood vessels within the eye can be valuable endpoints in a clinical trial. For instance, in individuals living with retinitis pigmentosa (RP), researchers have observed a reduction in the density of blood vessels and a relatively larger area in the retina where there are fewer or no blood vessels compared to normal retinal structures. 7
- Fundus Autofluorescence (FAF)
FAF is another non-invasive retinal imaging technology that highlights cell loss in the retinal layers. FAF works by using safe and low-intensity light to detect and capture signals from fluorophores, a naturally produced substance in the eye. Increased FAF signals may suggest areas of unhealthy retinal tissue; decreased FAF signals may suggest loss of cells in the retinal layers (atrophy). The quantity and quality of signals captured could be used as important endpoints in clinical trials, particularly in the classification of disease progression and recovery to measure treatment efficacy. 8 9
- Ultra-widefield imaging
Ultra-widefield imaging is a photography tool that uses a specialised camera to capture a broader view of the retina, as compared to traditional photography tools. This is very important in inherited retinal diseases, as many conditions initially cause damage to the peripheral parts of the retina (i.e. retinitis pigmentosa). Hence, ultra-widefield imaging allows a colour photograph and/or a FAF image to be captured out towards the edges of the retina, where the disease strikes first.
- Adaptive optics scanning laser ophthalmoscopy (AO-SLO)
AO-SLO (as pictured on the right) is an advanced non-invasive imaging system with a special laser used to scan a retina and create an incredibly detailed image. The “adaptive optics” part of the technology adjusts in real-time to correct any distortions or blurriness. Adaptive optics imaging transformed the landscape of exploring therapies for IRDs by providing higher image resolutions, enabling us to visualise individual retinal cells.10 This capability may allow accurate monitoring during the slow progression of IRDs, looking out for any tiny changes on a cellular level.
Currently, there is one commercially available AO system 11, but most of these cameras are found in research settings, such as the University of Melbourne.

2. Mobility testing
Most readers of this article will be aware of the commercially available gene therapy Luxturna, which was granted regulatory approval by the Australian Therapeutic Goods Administration (TGA) in 2020. Luxturna is a one-time gene therapy treatment for people with retinitis pigmentosa or Leber congenital amaurosis (LCA) from a mutation in the gene RPE65.
Excitingly for the research field, the approval of this drug by regulatory authorities relied heavily on “real-world” outcomes. In addition to showing that the treatment improved light sensitivity for people with the condition, the clinical trials also measured improvements on a mobility course, called the Multi-luminance Mobility Test (MLMT) 12 . Since this time, a number of other mobility tests have been developed.
In short, these tests are indoor mazes, where people need to walk through a set course, avoiding obstacles along the way 13 . The courses are completed at different light levels, which allows scientists to evaluate how effective the treatment is at improving night-time vision, vs daytime. A project to develop new mobility outcome measures was funded by Retina Australia in 2021, in a collaboration between optometrists, engineers and physiotherapists.
Whilst these mobility courses provide fantastic information on treatment efficacy, they are large (requiring significant space to setup), and time consuming for research participants. As such, work is underway to replicate the advantages of these courses using virtual reality devices, including through a project which is being currently funded by Retina Australia – Virtual Reality Assessment of Functional Vision in achromatopsia and albinism.

3. Patient reported outcomes
The other very important aspect of outcome measures in clinical trials is termed “patient reported outcomes measures (PROMS)”. In short, these are validated questionnaires that can be used to measure whether a person’s self-reported vision and/or their feelings about their vision have improved after treatment. Most PROMs are generic for cause of vision loss and used in many different types of clinical trials 14. However, there have recently been new PROMs developed that are specific for inherited retinal diseases (e.g. the Michigan Retinal Degeneration Questionnaire, MRDQ 15) or very people with very low vision (e.g. the Impact of Vision Impairment- Very Low Vision, IVI-VLV 16).
Whilst PROM questionnaires can take a lot of time to complete, and sometimes feel repetitive to those completing the survey, they provide essential information to scientists about the outcome of research and are an opportunity for the person participating to report their experiences.
How are we harnessing the potential of these technologies and endpoints?
The introduction of advanced imaging technologies has opened new avenues for assessing and understanding IRDs. Optical Coherence Tomography (OCT) and Optical Coherence Tomography Angiography (OCT-A) provide detailed insights into retinal structures and blood vessels, offering valuable information for evaluating treatment responses. Fundus Autofluorescence (FAF) and Ultra-widefield Imaging (UWF) contribute to the assessment of retinal health, providing crucial signals indicative of disease progression and recovery. Finally, adaptive optics provides the ability to image individual cells in the retina, which may allow us to identify changes at a much earlier time point than other photos can.
Mobility testing continues to be of importance in IRD clinical trials, and many studies are now including orientation and mobility instructors, occupational therapists and physiotherapists in the study design. Retina Australia’s current funding support for a research project in assessing the use of virtual reality assessment tools is also supporting these aims. New PROMS are also in development, including specific paediatric versions for children with IRDs.
Several projects are underway in Australia to learn more about IRDs and develop new outcome measures, including in New South Wales (Save Sight Institute IRD Registry), Western Australia (WARD study) and Victoria (VENTURE study). People with an IRD, or known carriers of such conditions, are strongly encouraged to register their interest in research through these registries, if applicable.
The recent notable setbacks in high-cost clinical trials failing to achieve their intended trial endpoints underscores the significance of selecting novel endpoints appropriate for slowly progressive IRDs and the critical importance of using the best technology and tools to do so. The success of cutting-edge technologies and further discoveries of state-of-the-art tools, with further identification of alternative outcome measures will continue to accelerate progress into IRD research.
https://retinaaustralia.com.au/accelerating-progress-in-ird-research-exploring-novel-endpoints-in-clinical-trials/
Other Blogs

World Geographic Atrophy Day 2025
A New Era of Hope for Vision Preservation Geographic Atrophy (GA), a progressive and irreversible form of advanced age-related macular degeneration (AMD), has long been a...

World Research Summary by Dr Catherine Civil
Hot Off The Press There are some exciting happenings in the research world this month. Click on the hyperlinks for further...

Australia Approves Second Treatment for Geographic Atrophy
Astellas' IZERVAY® (avacincaptad pegol) has been approved for use in Australia by the TGA Astellas...
